The metabolic engineering of Huazhong Agricultural University has modified yeast to efficiently synthesize glutathione
Glutathione (GSH) is a tripeptide thiol that plays a key role in cellular REDOX regulation and holds significant value in the fields of medicine and nutritional health products.
The traditional methods for extracting glutathione have many limitations, and the development of efficient microbial production systems has become a research hotspot.
Yeast has become an ideal host for the production of glutathione due to its excellent industrial compatibility and product tolerance.
To achieve the efficient synthesis of glutathione, a series of metabolic engineering challenges need to be overcome, including optimizing the synthetic pathway, enhancing enzyme activity, and increasing the supply of precursors.
September 23, 2025 Researcher Li Yingjun from the College of Life Sciences and Technology, Huazhong Agricultural University, published an article titled “Systems metabolic engineering of glutathione biosynthesis in” in the journal Engineering Microbiology Saccharomyces cerevisiae:
In the research paper “Pathway balancing coupled with enzyme screening for High-Energy production”, the team constructed an efficient glutathione biosynthesis platform in Saccharomyces cerevisiae through a systematic metabolic engineering strategy.
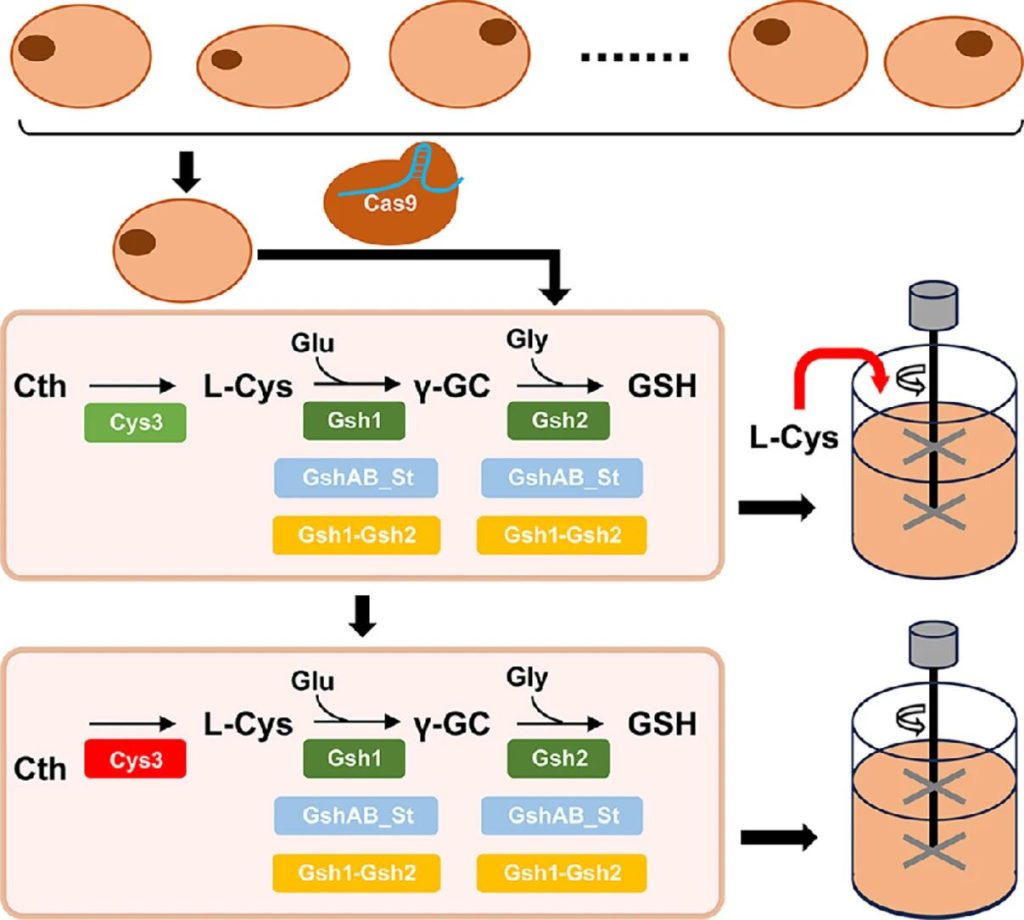
Researchers first screened out the Saccharomyces cerevisiae strain NJ-SQYY with superior glutathione accumulation capacity, and then used CRISPR/Cas9 technology to integrate the gshAB genes derived from different bacteria into the yeast genome.
Among them, gshAB_St from Streptococcus thermophilus performed the best, increasing the glutathione yield by 49.96%.
Through strategies such as promoter optimization and gene fusion, the synthesis capacity of glutathione was further enhanced, achieving a yield of 339.3 mg/L in shake flask culture, which is the highest level reported in chromosomal engineered Saccharomyces cerevisiae to date.
Dissolved oxygen coupled feed fermentation was carried out in a 5 L bioreactor, with a glutathione yield as high as 997.46 mg/L and a stem cell weight ratio of 33.85 mg/g.
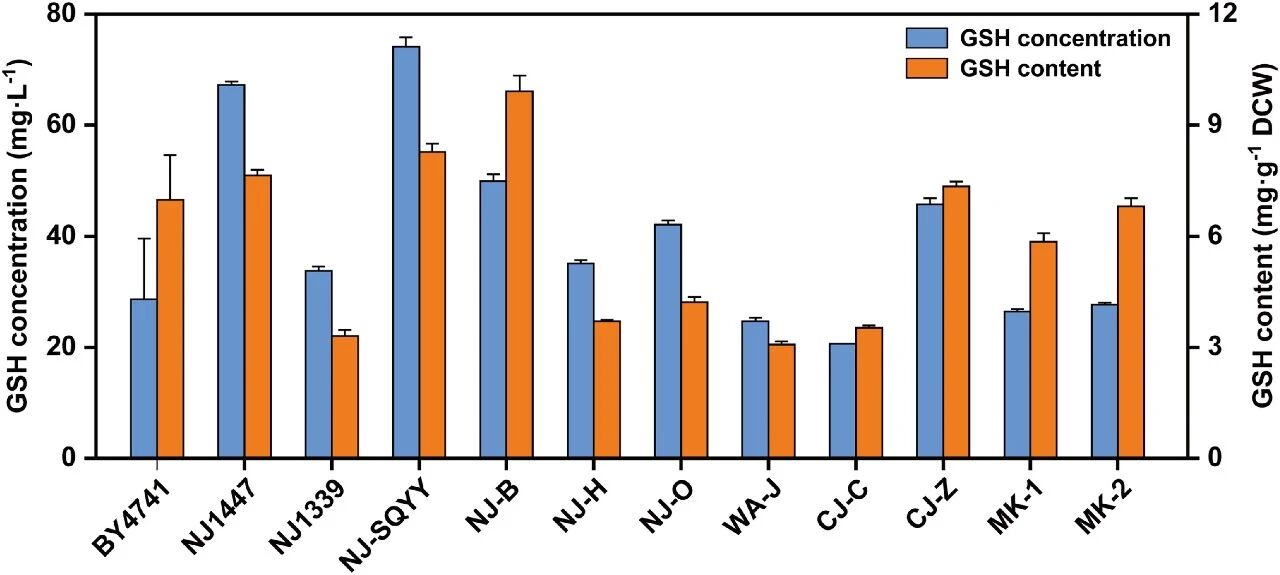
Researchers collected microbial samples from distiller’s grains, vinegar residue and fermentation agents in different geographical regions. After enrichment culture and category-specific separation, they finally screened out 31 strains of yeast, covering 24 different species.
Among these strains, Saccharomyces cerevisiae, Saccharomyces marskruviae and Candida lipophilis showed high levels of glutathione, while Pichia pastoris demonstrated superior biomass accumulation.
Through the culture analysis of 11 representative strains, it found that the glutathione titer of the NJ-SQYY strain the highest, reaching 74.14 mg/L, and the stem cell weight ratio was 8.27 mg/g.
Therefore, it was selected as the host strain for subsequent metabolic engineering modification.
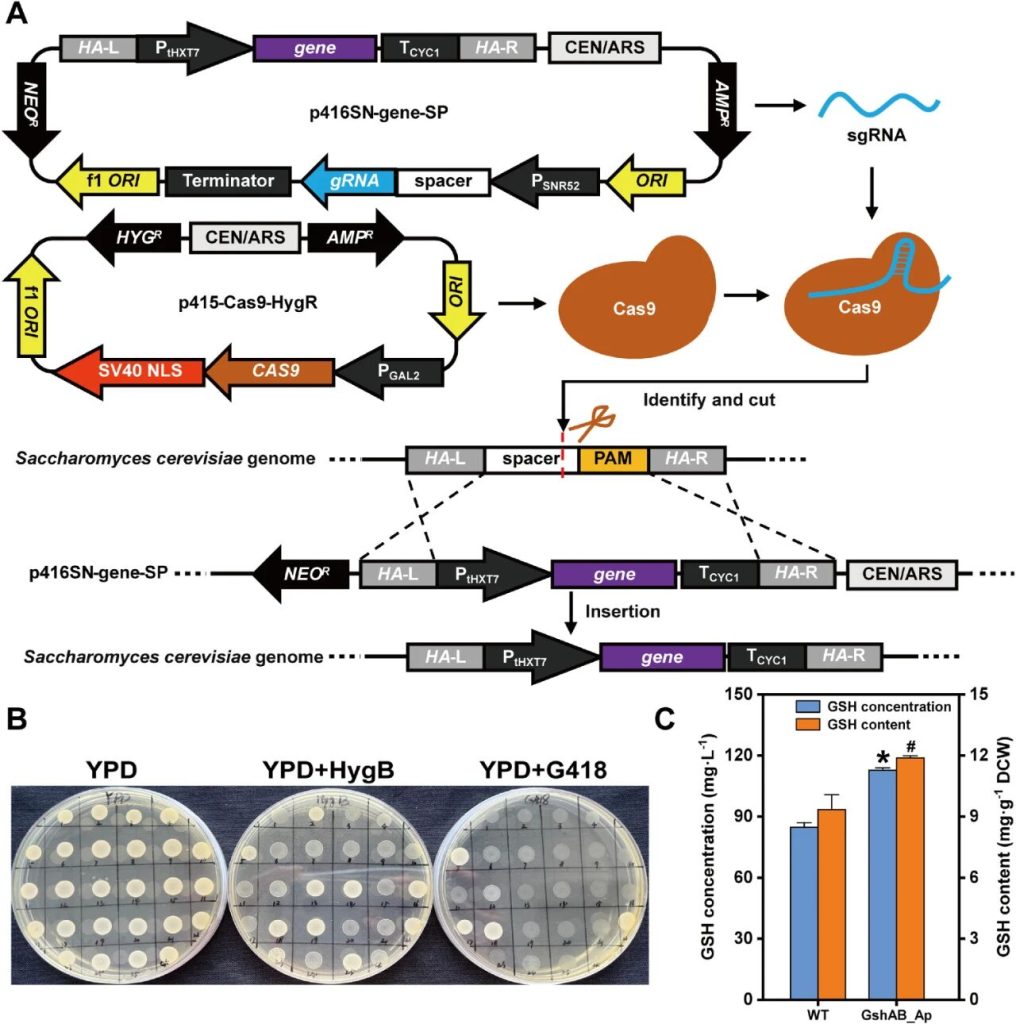
Researchers utilized the dual-plasmid CRISPR/Cas9 system to integrate the gshAB_Ap gene into the CS6 locus (YNCG0042C) of the NJ-SQYY strain.
The results showed that all eight selected clones exhibited the expected fragment size, confirming the high knock-in efficiency of the CRISPR system against Saccharomyces cerevisiae.
After successful integration, plasmid elimination is carried out through antibiotic-free culture to reduce the metabolic burden during the culture process.
The gshAB_Ap encoded by heterologous GshAB_Ap exhibited lower glutathione feedback inhibition than the natural yeast Gsh1.
Analysis showed that the glutathione yield of the SQ1 derivative significantly increased compared with the wild-type control.
The engineered strain showed a 33.14% increase in glutathione production (112.74 mg/L vs. 84.68 mg/L) and a 27.28% higher cellular glutathione content (11.89 mg/g vs. 9.34 mg/g).
The functional expression of the integrated gshAB_Ap and its positive metabolic effect on glutathione production demonstrated.
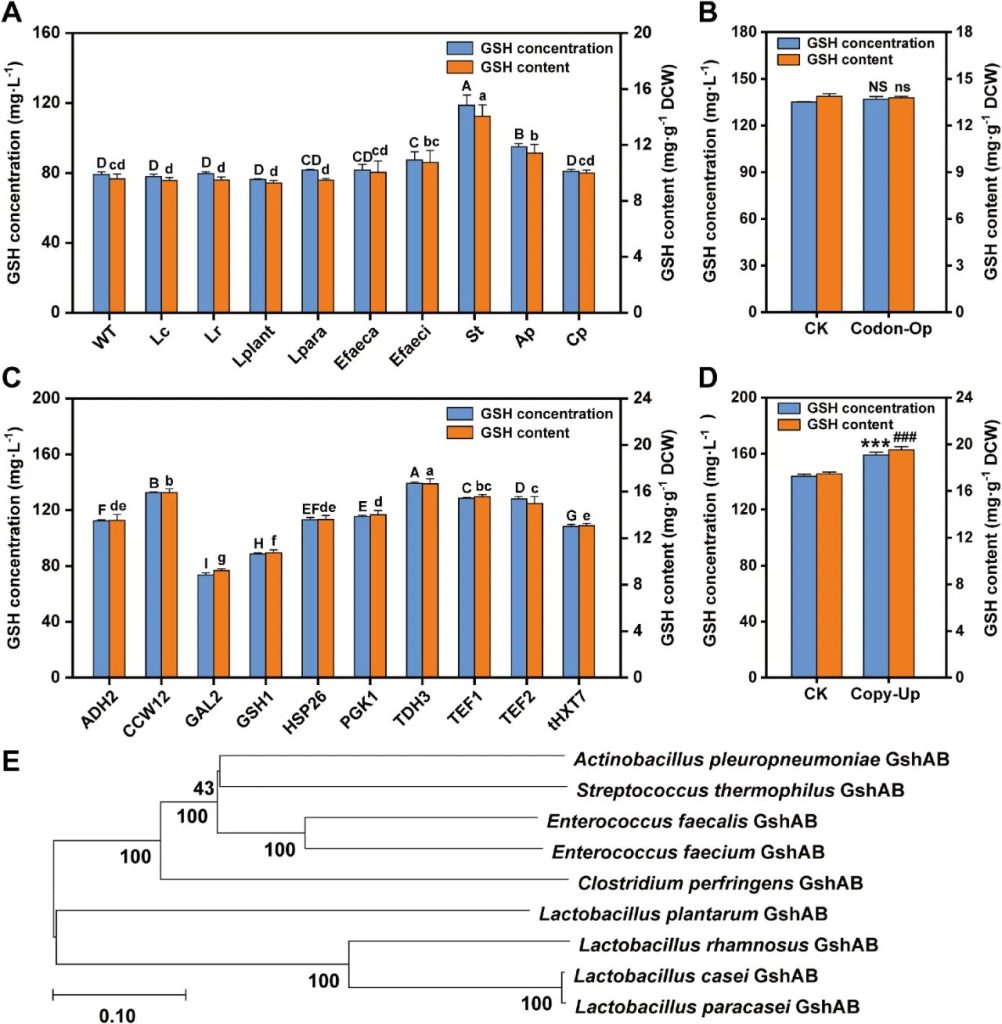
Researchers enhanced glutathione synthesis by heterologous expression of GshAB enzymes from different sources. They screened and collected gshAB genes from various Gram-positive bacteria from the NCBI database and integrated these genes into NJ-SQYY for expression.
The results showed that the strain SQ8 expressing gshAB_St from Streptococcus thermophilus performed the best, with a GSH yield of 118.65 mg/L, which was 49.96% higher than that of the wild type.
The GSH content of the cells reached 14.06 mg/g stem cell weight (DCW), which was 46.99% higher than that of the wild type.
Further strategies such as codon optimization, promoter engineering and increased gene dose employed to optimize the expression of gshAB_St.
The expression of gshAB_St driven by the PTDH3 promoter increased the GSH yield to 138.52 mg/L, which was 30.27% higher than that of the control group using the PtHXT7 promoter, and the GSH content in the cells reached 16.68 mg/g DCW.
Increasing the gene dose of gshAB_St through tandem integration only led to a limited increase in yield (10.44%) and content (11.72%), which might be related to the threshold of cell resource allocation and potential epigenetic silencing mechanisms.
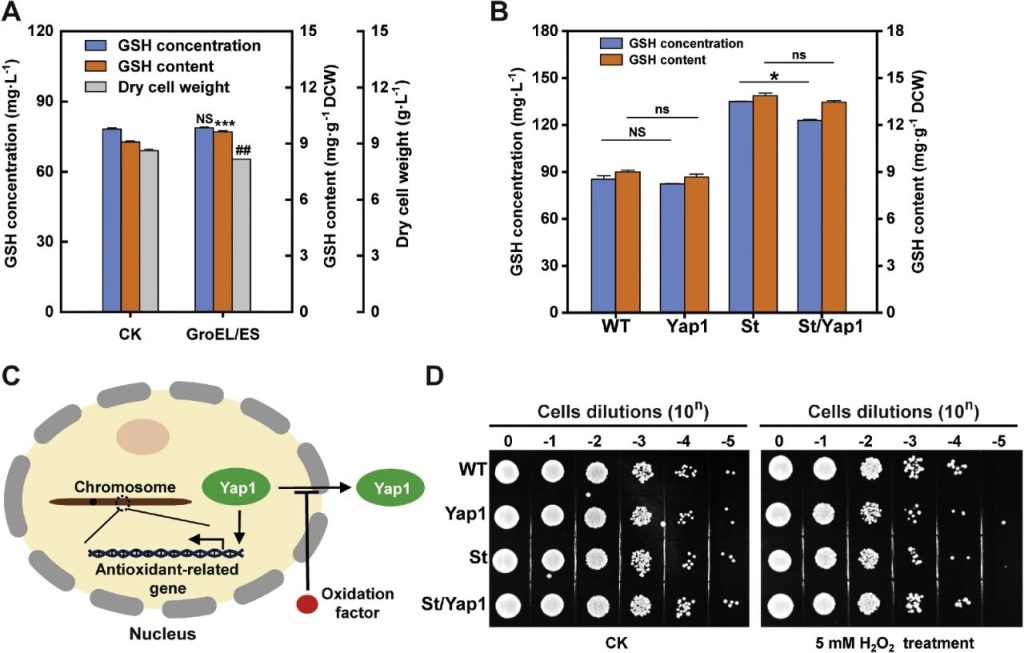
Researchers enhanced the system-specific restriction of glutathione synthesis in Saccharomyces cerevisiae through chaperone assisted folding and YAP1 transcriptional activation.
The molecular chaperone GroEL/ES of Escherichia coli introduced to assist in the folding of GshAB_Ap to address the issue of its heterologous expression in yeast.
Although this operation significantly increased the intracellular GSH level, it also led to a significant decrease in biomass, ultimately offsetting the increase in total GSH production.
Researchers attempted to enhance the synthesis of endogenous GSH by overexpressing YAP1, as YAP1 is the main transcriptional activator of oxidative stress in yeast and can regulate the expression of antioxidant defense genes.
The experimental results indicated that, whether in the wild-type NJ-SQYY or in the SQ8 strain expressing gshAB_St, the overexpression of YAP1 failed to increase the GSH level but instead led to growth inhibition.
This might because under standard culture conditions, the REDOX balance of cells is maintained, failing to trigger the nuclear translocation of YAP1 and the activation of antioxidant genes.
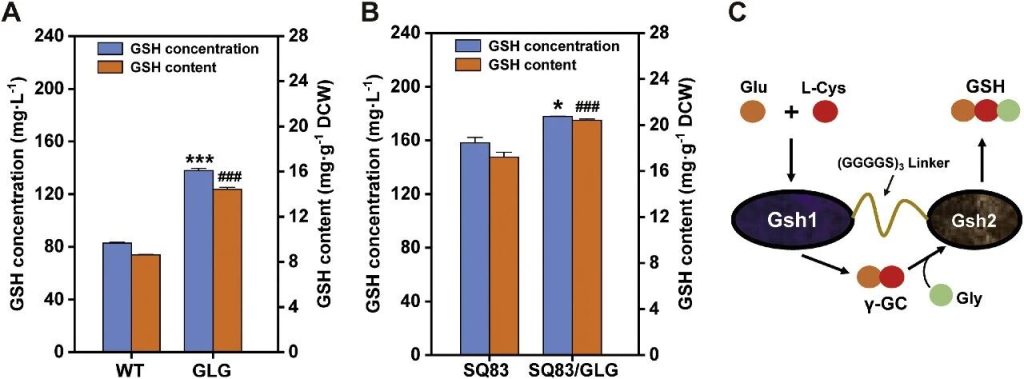
Researchers enhanced glutathione biosynthesis through enzyme fusion and promoter engineering. By using a flexible linker peptide GGGGS3, they linked the Gsh1 and Gsh2 enzymes to construct a bifunctional fusion protein (GLG), aiming to reduce the diffusion loss of intermediate products through substrate channelization and synchronize the enzyme expression ratio.
And the catalytic efficiency is improved through spatial co-localization.
This fusion protein integrated into the wild-type NJ-SQYY and the engineered strain SQ83, generating NJ-SQYY/GLG and SQ84, respectively.
The experimental results show that the GSH titer of SQ84 reached 177.76 mg/L, and the cell content was 20.41 mg/g DCW. Compared with SQ83, the yield and cell content increased by 12.33% and 18.64%, respectively.
The GSH titer and cell content of NJ-SQYY/GLG increased by 66.63% and 67.40% respectively compared with the wild type.
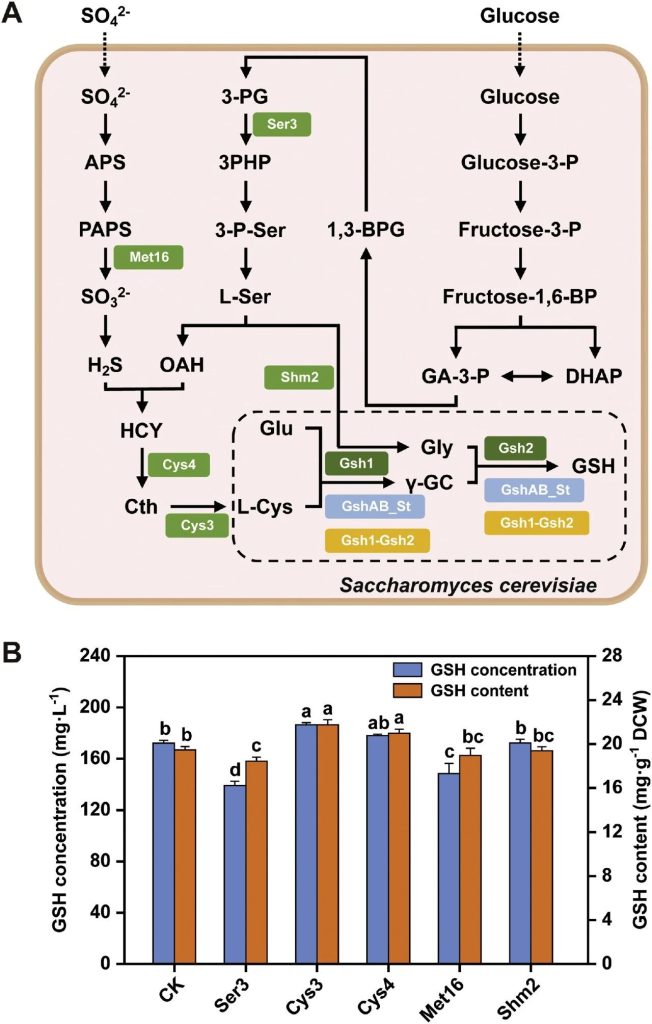
Researchers alleviated the metabolic bottleneck in the synthesis of GSH precursors by targeting the overexpression of key enzyme genes.
By analyzing the GSH biosynthetic pathway and based on literature evidence, five possible rate-limiting enzyme genes selected: Ser3, Cys3, Cys4, Met16 and Shm2.
These genes respectively integrated into the SQ84 background and expressed.
The experimental results showed that only the strain SQ842 overexpressing CYS3 significantly increased the titer and cell content of GSH, reaching 186.45 mg/L and 21.74 mg/g DCW, respectively. Compared with SQ84, they increased by 4.89% and 6.52%, respectively.
This indicates that the overexpression of CYS3 increases the supply of precursors for GSH synthesis by alleviating the bottleneck of L-cysteine (Cth) accumulation.
The overexpression of the other four genes (SER3, CYS4, MET16 and SHM2) did not increase the production of GSH, which might due to the fact that they restricted by redundant regulatory mechanisms or pathway cross-interactions in metabolic pathways.
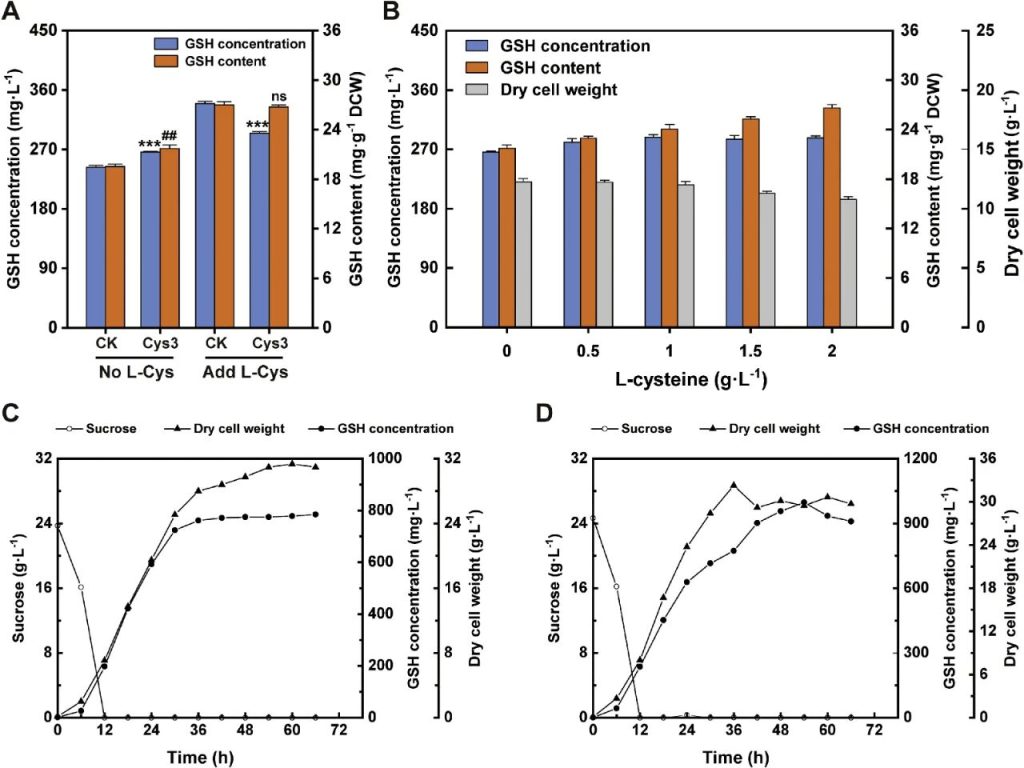
Researchers conducted a fermentation experiment of glutathione in a 5L bioreactor. During the feed fermentation process, SQ842 and SQ84 exhibit distinct growth and GSH production dynamics.
Under the condition of not adding exogenous L-cysteine, the biomass of SQ842 reached approximately 30 g/L, the GSH titer reached 784.85 mg/L at 66 hours, and the cell content was 25.37 mg/g DCW.
Conversely, under the condition of adding 2 g/L exogenous L-cysteine, the biomass of SQ84 reached the peak at 36 hours, the GSH titer reached 997.46 mg/L at 54 hours, and the cell content was 33.85 mg/g DCW.
This result indicates that SQ84 has a higher GSH production efficiency under the condition of adding exogenous L-cysteine, while SQ842 performs better without the addition of exogenous L-cysteine.
About GSH BIO-TECH